Silicon vs Carbon 硅元素与碳元素对比
Silicon vs Carbon 硅元素与碳元素对比
? A few important differences 主要不同点:
- The Silicon atom is larger 硅原子更大
- Silicon is less electronegative 硅原子很少带负电
- Silicon does not form multiple bonds硅不能组成多种键
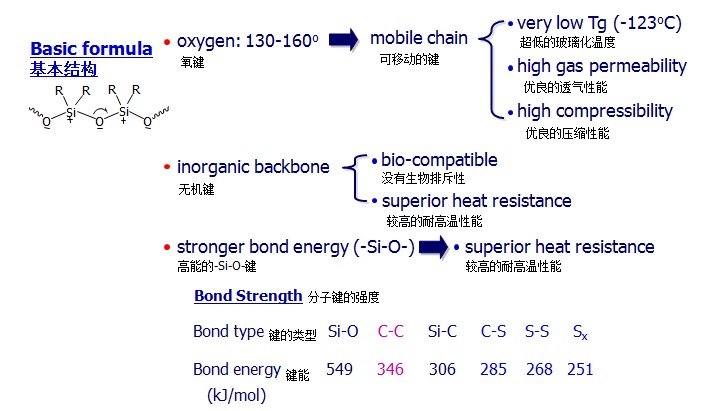
- Bond Energies 键能:
C - C 83 kcal / mole 83千卡/摩尔
C - O 86 kcal / mole 86千卡/摩尔
Si - Si 46 kcal / mole 46千卡/摩尔
Si - O 106 kcal / mole 106千卡/摩尔
? Bond Lengths 键长:
C - C 1.5 angstroms 1.5埃
C - O 1.4 angstroms 1.4埃
Si - O 1.6 angstroms 1.6埃
? Bond Angles 键角:
C - C - C 109 degrees 109度
C - O - C 111 degrees 111度
Si - O - Si 130 degrees 130度
? Despite the fact that Silicon and Carbon are both Group IV elements, their chemistry is very different: 虽然硅和碳同是IV组元素,但是属性有很大不同:
CO2 is a simple gas SiO2 is a complex solid polymer
二氧化碳是简单的气体 二氧化硅是复杂的固体
CCl4 is a reasonably stable fluid SiCl4 is highly reactive to water and many organic substrates
四氯甲烷是比较稳定的液体 四氯硅烷容易和水、多数官能团发生反应

Properties of PDMS 聚二甲基硅氧烷的特性
Poly-dimethyl-siloxane 聚二甲基硅氧烷
- Inorganic Backbone High Surface Energies
- 无机分子键 高表面能
- Pendant organic (methyl) groups Low Surface Energies
- 有机分子键 低表面能
Role 在硅橡胶的作用:
- Backbone presents the organic groups at the interface
- 无机键在内层,有机键在外层
- Organic groups provide the Surface Active properties
- 有机官能团提供表面活性

Chemistry of HTV silicone rubber HTV的化学特性